Repurposed generics could be a game changer for patients
By Emily Zhu
Cancer patients urgently need more effective and affordable treatments. Repurposing of drugs that are safe, available, and inexpensive could immediately help cancer patients around the world live longer and better.
What is Drug Repurposing?
Drug repurposing is investigating whether a drug that has been approved by a regulatory agency to treat one disease is also effective for treating a different disease. As shown in the table below, many common treatments were originally developed for a different indication and were then repurposed.
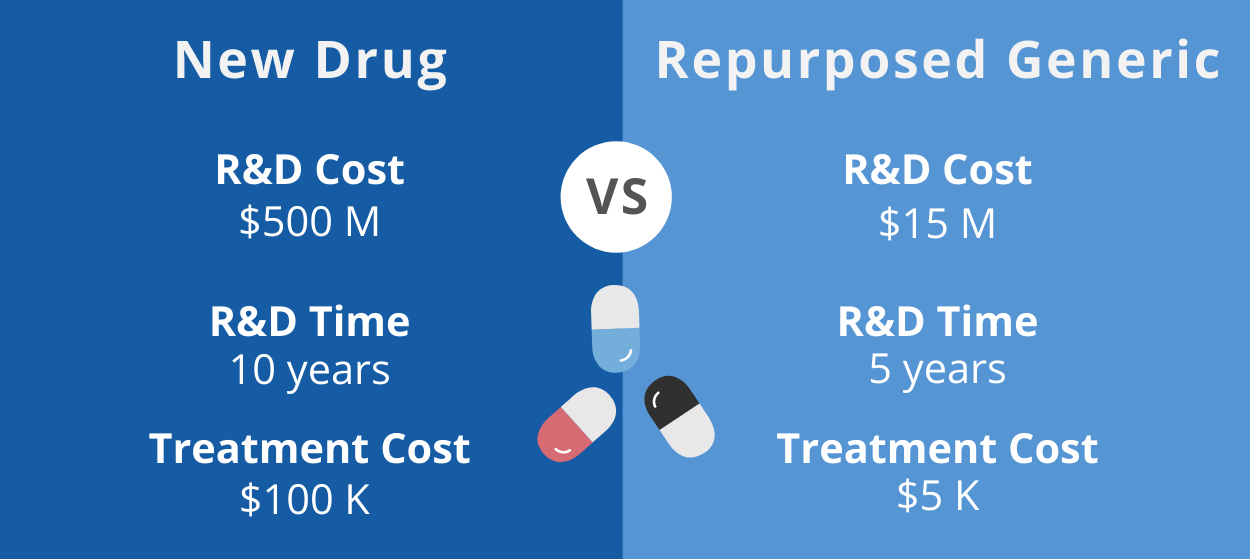
Repurposing off-patent generic drugs as-is (without changing the drugs in some way, such as through reformulation) is the fastest way to develop new and affordable treatments. Pharmaceutical companies receive patent protection and market exclusivity for around 20 years for a newly developed drug. After the patent expires, other manufacturers can make and sell a generic version of the drug, which drives down the drug price due to competition in the marketplace.
How Generic Drug Repurposing Can Help Cancer Patients
Repurposing of generic drugs has increasingly become recognized as the most efficient way to develop new treatments. A new drug takes more than 10 years and up to $2 billion to gain FDA approval due to the long research and development (R&D) process and the large fraction of drugs that fail during this process because of either efficacy or safety concerns. On the other hand, early steps in the R&D process (such as preclinical studies and Phase I/II trials) can often be skipped for repurposed drugs, and these drugs are less likely to fail during clinical testing for safety reasons since they have a long history of safe patient use. A definitive clinical trial to prove the efficacy of a repurposed drug could cost less than $10 million and transform the standard of care for cancer within 5 years.
Repurposed generic drugs can also decrease cancer care costs. In the 1990s, the median cost of cancer drugs was less than $100 per month; by 2011, the cost had risen to around $10,000 per month. In contrast to these expensive on-patent drugs, off-patent generic drugs can cost as little as a few hundred dollars a month.
Successful Drug Repurposing for Cancer
Three non-cancer generic drugs are now the standard of care for cancer:
The acne treatment all-trans retinoic acid (ATRA) was repurposed to treat acute promyelocytic leukemia (APL). ATRA helps the promyelocytes affected by APL develop into fully functioning cells (neutrophils). Approximately 70 to 80 percent of APL patients go into remission after being treated with ATRA in combination with chemotherapy.
The tuberculosis prevention vaccine Bacillus Calmette-Guerin (BCG) replaced cystectomy as the standard of care for bladder cancer in the 1980s. BCG is an immunotherapy that is proven to reduce the risk of recurrence. Ongoing BCG therapy reduces the risk of progression in patients with high-grade non-muscle invasive bladder cancer.
Thalidomide, a drug that was previously marketed as a treatment for pregnancy-related morning sickness and later recalled due to its teratogenic effects on fetuses, was approved for treatment of plasma cell myeloma in 2006. Thalidomide induces immune responses, enhances the ability of immune cells to kill myeloma cells, inhibits inflammation, and blocks the formation of blood vessels which prevents tumor growth.
Scaling Generic Drug Repurposing
More than 250 generic drugs, FDA-approved for non-cancer indications, have shown promise for treating cancer in preclinical or early clinical studies. These repurposed generic drugs could improve cancer outcomes while helping relieve the financial burden that cancer patients and their families face. Yet they are not being tested for cancer in definitive clinical trials due to the lack of funding for repurposing off-patent, inexpensive drugs.
Our nonprofit Reboot Rx is developing innovative approaches to be able to scale generic drug repurposing and get more affordable treatments to cancer patients more quickly.