Reboot: COVID-Cancer Project: a proof-of-concept of our evidence synthesis technology
By Laura Kleiman, PhD, Founder and CEO of Reboot Rx
At Reboot Rx, we are fast-tracking the development of affordable cancer treatments using repurposed non-cancer generic drugs and AI technology. To determine which drugs are the most promising to repurpose for cancer, we need to review data from hundreds of thousands of published studies. These are studies that have already been conducted that might contain information about the efficacy of non-cancer generic drugs for the treatment of cancer. It’s like looking for a needle in a haystack. It would take a lifetime to find the highest quality studies and understand the patterns in the data. We are solving this challenge by building technology that uses AI and machine learning to quickly sift through large amounts of data. Our technology synthesizes cancer data for non-cancer generic drugs to help us find the most promising repurposing opportunities.
Motivations for the Reboot: COVID-Cancer Project
We were underway developing our technology when the coronavirus pandemic hit. It was soon discovered that patients with certain cancers are a particularly vulnerable population and are up to three times more likely to die of COVID-19 than those without cancer. It became clear that cancer patients who contract COVID-19 and their doctors would have an urgent need to find information about potential treatments.
The public health crisis caused by COVID-19 has propelled drug development forward at an unprecedented rate. This has led to an explosion of data on the effects of COVID-19, and its potential treatments, for cancer patients. It became nearly impossible to quickly find the most helpful information. We knew our technology could accelerate the search for the most relevant data.
Our technology can be used to research all types of drugs - non-cancer generics or otherwise. Most of the drugs being tested for COVID-19 are repurposed generics. Coincidentally, some of these drugs have also been tested for treating cancer. It made sense to use our technology to pull together the data showing how potential COVID-19 treatments might affect a patient’s cancer.
Saving time, saving lives
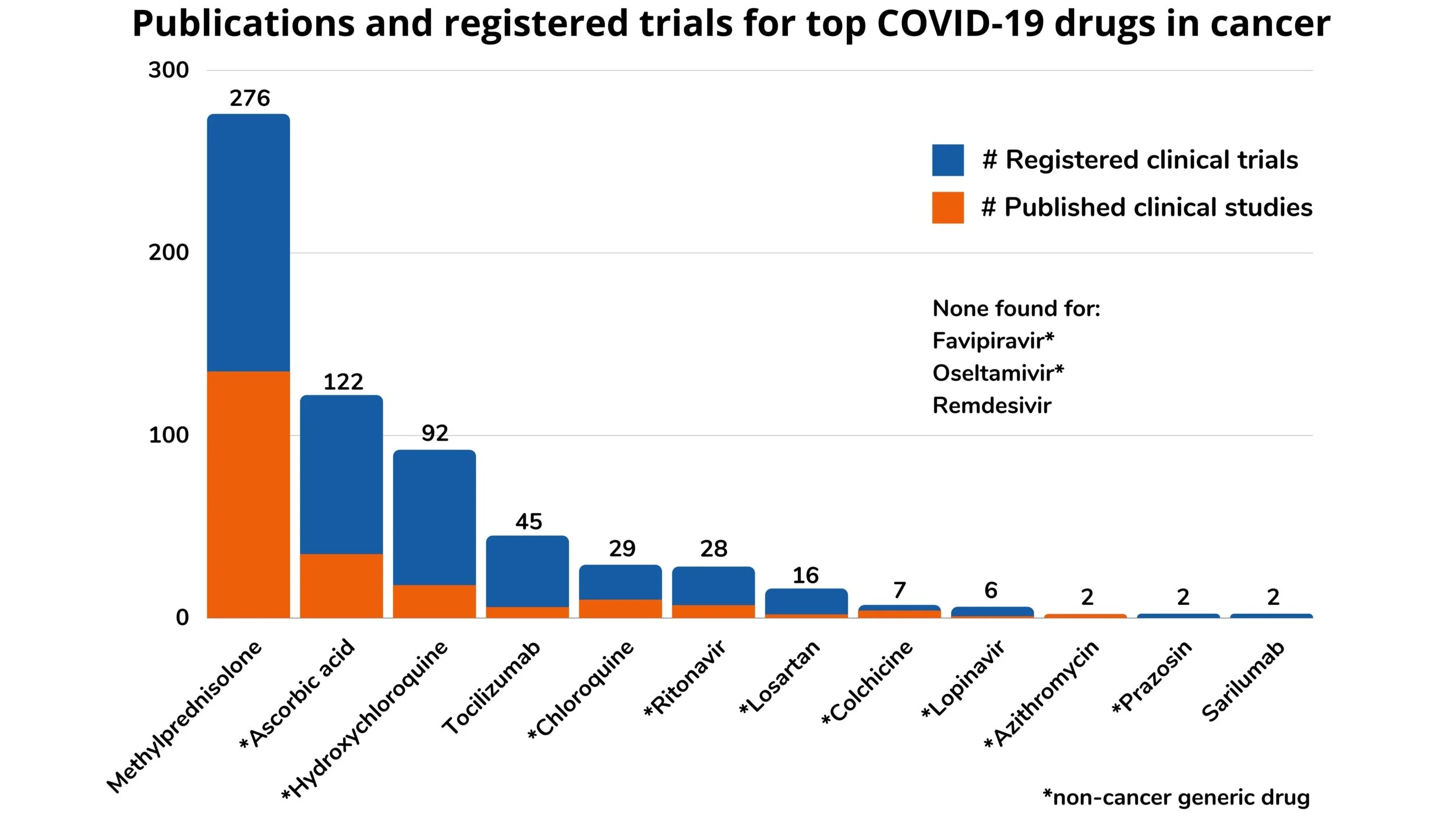
We began the Reboot: COVID-Cancer Project in April 2020. We applied our technology to rapidly narrow in on the most relevant studies on COVID-19 and cancer, which reduced the time needed for us to manually review studies from 1 year to 5 weeks. We identified 915 clinical studies related to COVID-19 and cancer from nearly 200,000 publications and registered clinical trials. From the relevant studies, we extracted information such as the cancer type, drug tested, study type, and outcomes.
We released free, interactive dashboards for easy access to two datasets. The first dataset draws from recent studies on COVID-19 in cancer patients. The second dataset draws on years of cancer research on drugs that are now being tested for COVID-19. The dashboards are being used by doctors treating cancer patients with COVID-19 and by researchers to accelerate the search for effective treatments.
A proven and scalable strategy
There’s more information on the potential effects of non-cancer generic drugs on cancer than one might think. There are around 1,500 non-cancer generics. These drugs were FDA-approved for non-cancer indications and are now off-patent. As part of the Reboot: COVID-Cancer Project, we started with 11 of these that are being tested for the treatment COVID-19. For just these 11 non-cancer generics, using our technology we found 78 published clinical studies (clinical trials, observational studies, meta-analyses, and case studies) and 225 registered clinical trials (trials previously conducted or actively in progress) where these drugs were tested for the treatment of cancer. Extrapolating from this ratio, we believe there are around 10,000 published clinical studies, plus many more preclinical studies, for all 1,500 non-cancer generic drugs.
The value of this work is not only that we helped cancer patients with COVID-19 by making this information readily available for people who urgently need it, but we also proved that our technology works. We increased the size of our training datasets, refined our pipeline, and improved the performance of our machine learning models - and ultimately demonstrated how our technology could have immediate positive impact.
Moving forward
We are now scaling this effort to synthesize the cancer data for all 1,500 non-cancer generic drugs, with the goal of identifying the most promising generics to repurpose for cancer. We'll then bring together the various stakeholders to run definitive clinical trials and change the standard of care. Our technology is accelerating the critical data review step so we can get effective treatments to patients faster.